A new modular automation system for clinical trials drug delivery – now in operation at the Medical Manufacturing Innovation Centre (MMIC) in Strathclyde – is giving pharmaceutical and life sciences a game-changing insight into a future where new products can be delivered faster, more flexibly and cheaper than before.
The disruptive, patented technology is manufactured by British firm CME Automation Systems and is known as PACE, which stands for Pharmaceutical Automation for Clinical Excellence.
PACE fulfils the need defined in the CPI’s Grand Challenge 2 for ‘Delivering Automated Just-In-Time Clinical Supply.’ It does this by enabling the manufacture of multiple products at a smaller scale, enabling faster changeovers and late-stage customisation while maintaining tight clinical protection.
For the clinical trials industry, PACE opens the door for more trials per year, as well as more diverse trials – for example, by trialling multiple doses and strengths simultaneously.
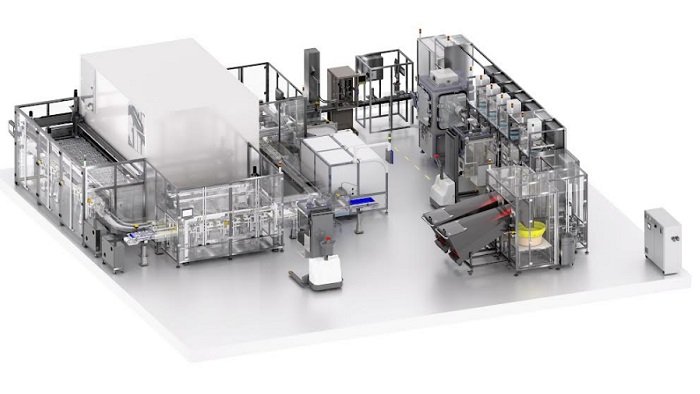
The system fills pill bottles with different types of drugs, in tablet, capsule or vial format, without the risk of cross contamination, whilst maintaining complete traceability throughout the system. With a modular design that allows multiple self-contained chambers to be incorporated, it significantly reduces the time taken to bring new medicines to market.
“Clinical trials need to be carefully controlled at every stage, and that inevitably makes them expensive and time-consuming to run. One of the biggest issues facing pharmaceutical manufacturers is how to maintain a cleanroom environment during packing – and that’s what our patented PACE technology does,” explained Paul Knight, CEO of CME Automated Systems.
“This unlocks true JIT capabilities – multiple small runs can be delivered quickly under cleanroom conditions,” he added.
A key innovation is the patented design of the filling station. This enables a single line to incorporate a series of subsequent humidity and temperature-controlled drug filling stations, each configured as a fully functioning independent unit. They are designed to allow quick changeover of pharma product types, in order dispense tablets or capsules of differing drug types and strengths.
Crucially, while of course the whole packing line still needs to be in a cleanroom environment, only one cleanroom is now required to pack multiple drug types and formats.
The filling stations sit at the centre of a full line solution, comprising handling, filling, sealing, weighing, marking, labelling and packing. It is digitally enabled to provide real-time connectivity for greater accuracy and speed in verification, via QP dashboard, and ongoing data insight using digital twin technology. Rigorous checking processes along the line reject any bottle that fails and these are logged so that that specific order can be fulfilled at the next available opportunity.
With funding from CPI and major pharmaceutical multi-nationals, the world’s first PACE system is running at MMIC. Initial trials by some of the centre’s leading investors are underway to provide invaluable data on the performance of the system.
The investment in PACE by CPI aligns with the government-backed organisation’s Grand Challenge 2: Delivering Automated Just-In-Time Clinical Supply. The stated ambition is to develop a supply chain of the future which can drastically reduce current timescales of over 300 days to 30 days.
According to CPI, “A key innovation in PACE enables it to deliver the production, packaging and labelling of multiple drugs in the same facility without cross-contamination. It all takes place on a single line in a GMP environment with real-time quality checks, resulting in less waste, risk and cost while maximising speed to patient.”
Paul Knight confirmed: “We’re delighted to be part of such an important initiative and look forward to welcoming interested parties to Strathclyde to see PACE in operation at MMIC.”